The U.S. Drug Enforcement Administration (DEA) has launched a new tool to assist drug manufacturers and distributors meet their regulatory obligations under the Controlled Substances Act.
This new resource, an added feature to the ARCOS Online Reporting System, is an example of the many ways DEA is working collaboratively with its 1.73 million registrants to combat the ongoing opioid epidemic in the United States.
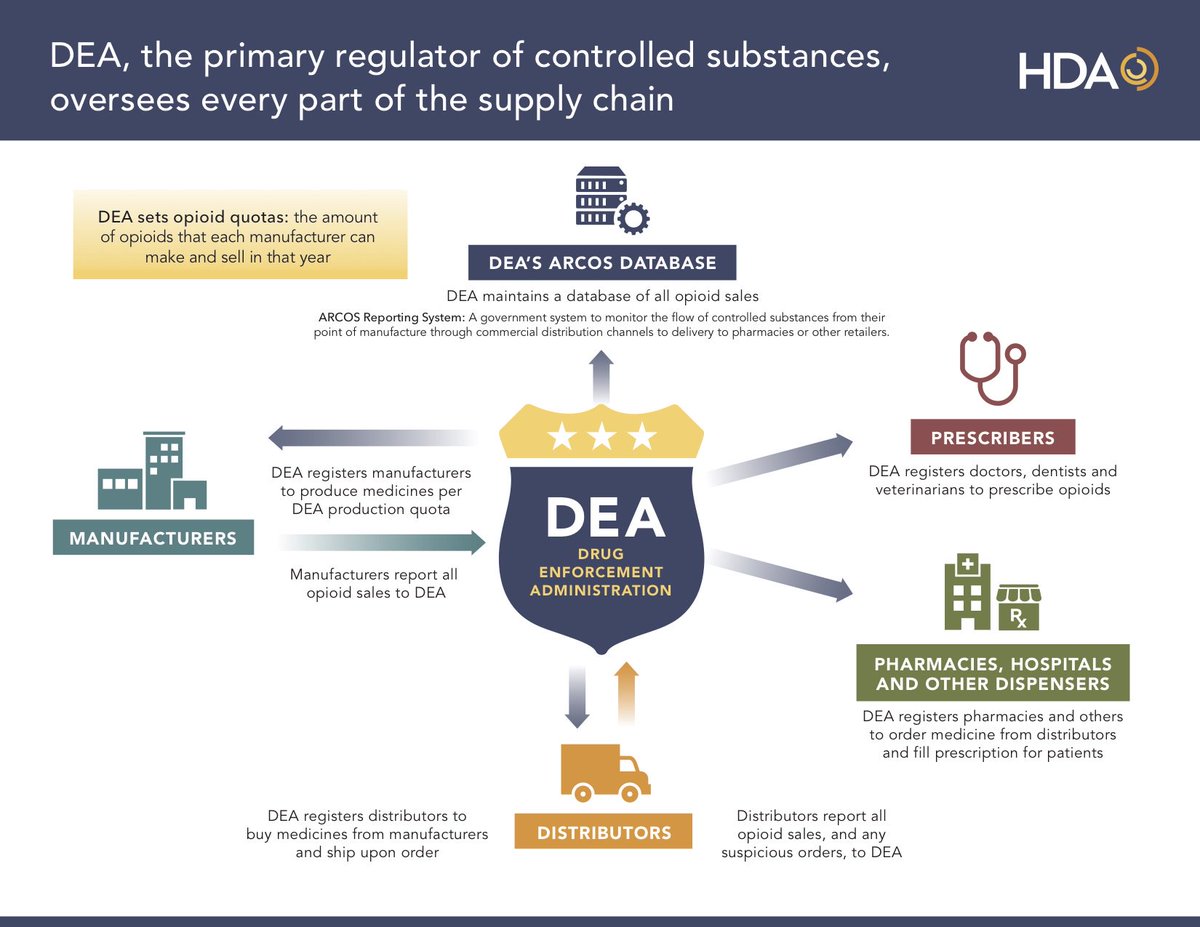
ARCOS is an automated, comprehensive drug reporting system which monitors the flow of controlled substances from their point of manufacture through commercial distribution channels to point of sale or distribution at the dispensing/retail level – hospitals, retail pharmacies, practitioners, mid-level practitioners, and teaching institutions.

Included in the list of controlled substance transactions tracked by ARCOS are the following:
- All Schedules I and II materials (manufacturers and distributors)
- Schedule III narcotic and gamma-hydroxybutyric acid (GHB) materials (manufacturers and distributors); and
- Selected Schedule III and IV psychotropic drugs (manufacturers only)
Furthermore, the DEA recently issued a temporary scheduling order to schedule all fentanyl-related substances, not currently listed in any schedule of the Controlled Substances Act (CSA) in schedule I, to reduce these substances’ flow into the country and slow the alarming increase in overdose deaths linked to synthetic opioids. (See below to learn more.)
ARCOS accumulates these transactions which are then summarized into reports which give investigators in Federal and state government agencies information which can then be used to identify the diversion of controlled substances into illicit channels of distribution.
The information on drug distribution is used throughout the United States (U.S.). by U.S. Attorneys and DEA investigators to strengthen criminal cases in the courts.
This newly added function will allow the more than 1,500 DEA-registered manufacturers and distributors to view the number of competitors who have sold a particular controlled substance to a prospective customer in the last six months.
DEA regulations require distributors to both “know their customer” and to develop a system to identify and report suspicious orders.
Manufacturers and distributors have consistently expressed a desire for assistance from DEA in fulfilling these obligations and have requested ARCOS information to help them make informed decisions about whether new customers are purchasing excessive quantities of controlled substances.
This new tool will provide valuable information for distributors to consider as part of their assessment.
For example, if a query resulted in a large number of suppliers who have recently sold opioid analgesics to a prospective purchaser, this may represent a “red flag” to the new distributor and foster a dialogue between that distributor and the pharmacy.
To register for the program, the following ARCOS EDI Request Form must be completed and faxed or mailed to the Drug Enforcement Administration.
Fax the completed form to the ARCOS Unit at 202-307-8612 or mail it to DEA Headquarters, Attn: ARCOS Unit, P.O. Box 2520, Springfield, VA 22152-2520.
When the DEA receives the EDI request form, the ARCOS Unit will contact the responsible individuals to set up user accounts and provide all information needed for participation in the program.
Please direct questions regarding the ARCOS EDI program to the ARCOS staff at (202) 307-8600 or ARCOS_Unit@usdoj.gov for email inquiries.
Learn More…
DEA Emergency Schedules Fentanyls to Reduce Overdoses (Multi-Video)


















